UNDERSTANDING REBIF®
Jump to topic:
In order to choose the treatment that’s right for you, it’s important to understand a little about your type of multiple sclerosis (MS) and what to expect from your medication. Rebif® (interferon beta-1a) is a treatment for relapsing forms of multiple sclerosis (RMS), the most common type of MS.
RMS is an autoimmune disorder in which the body attacks itself. Specifically, the immune system attacks the coating (the myelin sheath) on the nerves in the brain, spinal cord, and eye area, which can cause scarring and prevent the nerves from communicating correctly. This communication breakdown can lead to symptoms of MS, which can vary in severity, and may lead to permanent damage.
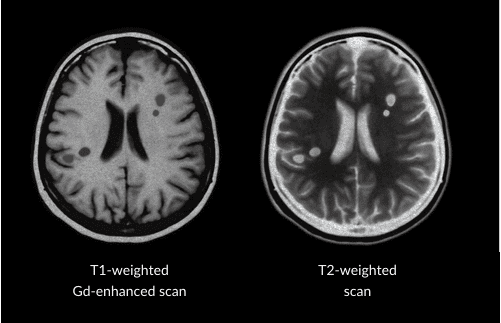
When the body’s immune system attacks the covering of the nerves, it leaves scars called lesions. Technically, these are referred to as “sclerotic lesions,” which is where MS gets its name. Healthcare providers look for these lesions using magnetic resonance imaging (MRI) to help them diagnose RMS and select an appropriate treatment for their patients. Once a patient is taking a treatment, healthcare providers use MRI scans to judge how well that treatment is working. Rebif® (interferon beta-1a) has been proven in a clinical trial to reduce the number of new and enlarging MRI brain lesions—this is another one of the important goals of treating RMS.
They use MRI scans.
MRI machines use magnets to see inside the brain and spinal cord to find lesions. There are 2 types of MRI scans usually used for people living with RMS. A T1-weighted gadolinium-enhanced (T1-Gd+) MRI supplies information about current disease activity by highlighting areas of active inflammation and permanent nerve damage, whereas a T2-weighted MRI image provides information about total disease burden (that is, both old and new lesions).
RMS is characterized by relapses and remissions.
Relapses—also called flare-ups, attacks, or exacerbations—occur when 1 or more RMS symptoms (such as numbness or blurred vision) appears or worsens, and remains for at least 24 hours
During the remission phase, the symptoms may partially or completely go away
In the chart below, you can see that a person’s abilities may not fully recover following a relapse.
Note that being in remission does not mean you no longer need your treatment. It is important to continue Rebif® as prescribed by your healthcare provider
Rebif® does not treat the symptoms of RMS, but has been proven in a clinical trial to help reduce the frequency of relapses. See study results showing reduced relapse rates with Rebif®
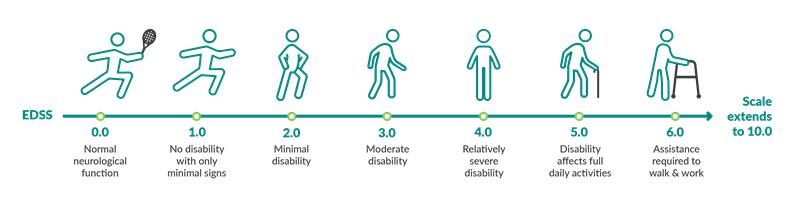
Disability progression is measured using the Expanded Disability Status Scale (EDSS). This is one method healthcare providers use to measure disability progression, especially in the clinical trial settings.
Here’s how it works: After examining and assessing the patient, a healthcare provider assigns a number based on their level of disability. That score can be compared at a later date to measure any progression in the patient’s disability.
People who have EDSS scores from 1 to 4 are fully ambulatory, meaning they can still walk. For people with scores 5 or higher, their ability to walk will be increasingly impaired. In clinical trials, comparing scores over time can help researchers judge how well a treatment helps to slow disability progression—an important treatment goal.
Rebif® is a form of interferon beta, an immunomodulator which helps to regulate the immune system. Rebif® is similar to the type of interferon beta produced naturally in your body. When you have RMS, treatment with an interferon therapy may reduce the activity of cells that are attacking your nervous system.
The precise way in which Rebif® works in MS is not known. Talk to your healthcare provider to find out what Rebif® may mean for you and your RMS.
IMPORTANT SAFETY INFORMATION AND INDICATION
Important Safety Information
Do not take Rebif if you are allergic to interferon beta, human albumin, or any of the ingredients in Rebif.
Rebif can cause serious side effects. Tell your healthcare provider right away if you have any of the symptoms listed below while taking Rebif.
Before you take Rebif, tell your healthcare provider if you have or have had any of the following conditions:
Tell your healthcare provider about all medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
The most common side effects of Rebif include:
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Rebif. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
Indication
Rebif® (interferon beta-1a) is a prescription medicine used to treat relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. It is not known if Rebif is safe and effective in children.
Please see Rebif® Prescribing Information and Medication Guide.